Comparison of four heavy metal wastewater treatment technologies (Heavy Metal Treatment Part II)
Are you evaluating the treatment plan, have you chosen the right method?
Facing the ever-changing electroplating process wastewater, choosing the right wastewater treatment plan is half the battle!!!
Focusing on four common heavy metal treatment technologies, we discuss the technology through practical experience and analyze the difficulties and countermeasures of heavy metal treatment~
"Why do the heavy metals in the discharged water exceed the standard again? We have done all the medicines and new equipment that should be added, but why are they still not up to standard?" The treatment efficiency is not good, but there are always compromises or no solutions. This is what we often do on site. What happened. In fact, there are many reasons why heavy metals do not meet the standards. The initial treatment technology selection, changes in process wastewater composition, chemical selection and addition amount, etc., will all affect the final treatment results.
Generally, in related industries, such as the metal surface electroplating industry or the electroplating process of electronic components, a certain amount of metal wastewater will be generated due to the aging of the tank solution or the cleaning liquid. There are usually several methods to choose from when dealing with this type of metal wastewater. Each has its own advantages and limitations. Appropriate treatment options must be selected according to the characteristics of each plant's wastewater. The following summarizes and introduces four common heavy metal treatment technologies, discusses the technology through practical experience, and analyzes the difficulties and countermeasures of heavy metal treatment. Finally, the characteristics, advantages and disadvantages of each technology are compared, and a selection process is designed to allow decision-makers to easily judge and adopt a solution that better meets their needs during the initial evaluation.
.jpg)
▲Selecting the appropriate treatment solution is the primary factor for stable performance of the wastewater treatment system
Four common heavy metal treatment technologies include alkali precipitation, heavy metal treatment, ion exchange and electrolysis. According to the treatment technology, the alkali precipitation method and the recapture agent treatment method are chemical methods, which mainly rely on dissolving metal ions and OH, S, or S derivatives in water to form insoluble metal precipitates, and then through coagulation, gelation, and precipitation. and other steps to remove the sediment from the water to obtain effluent water with low metal concentration.
The other two methods are ion exchange resin and electrolysis, physical adsorption and chemical treatment respectively. The physical adsorption method mainly uses heavy metal ions in the water to exchange with ions with relatively weak affinity in the resin, and then uses desorption to discharge the concentrated heavy metal ion concentrate with higher concentration and the treatment liquid with lower concentration. Electrolysis uses the positive electrode to provide electrons for oxidation reactions, while the negative electrode obtains electrons for reduction reactions, reducing heavy metal ions in the water into solid metals and depositing them on the electrode plates, thereby reducing the metal concentration in the water.
Alkali precipitation (Metal Hydroxide Precipitation)
It is the main treatment method currently used, and its use conditions are limited by the influence of environmental conditions (such as pH). This method makes use of the characteristics of amphoteric metals, which have the lowest solubility within a certain range, that is, the metals that form hydroxides have the most solid precipitation at this pH value. And as the pH gets higher, the metal hydroxide precipitate gradually dissolves in water in other forms. The figure below shows the theoretical relationship between the pH value of each metal and the metal hydroxide.
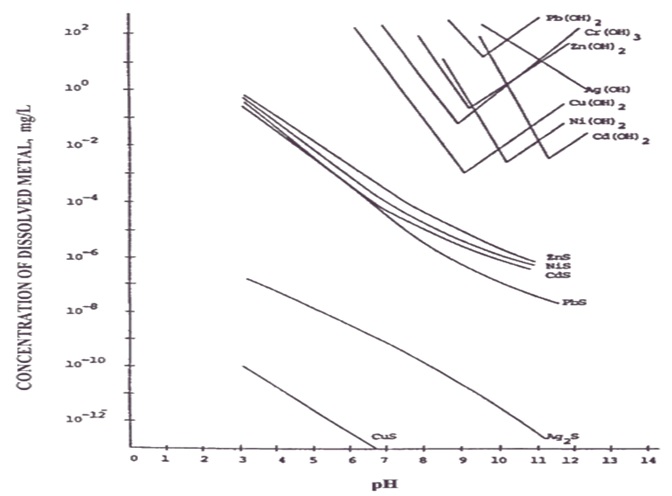
(Source : Physicochemical Treatment Processes-Handbook of Environmental Engineering)
Based on this relative relationship diagram, on-site operations will consider the characteristics of individual wastewater and the presence of single or multiple metals to determine the most suitable pH range, so it is often not at the lowest point of the curve in the diagram. For example, in a nickel electroplating wastewater treatment plant in Taichung, according to on-site operation experience, the optimal pH is around 11. Under this condition, the most obvious glue feathers can be produced, and the nickel concentration in the discharge water is also the lowest. The following table summarizes the optimal precipitation operating range and theoretical minimum concentration of each metal based on actual field experience:
| Type of metal | pH range for precipitation | Theoretical min. level in water(mg/L) |
| Nickel | 10-11 | 0.003 |
| Zinc | 9-10 | 0.1 |
| Copper | 8.5-9.5 | 0.001 |
| Chromium | 8.5-9.5 | 0.3 |
| Cadmium | 11-12 |
0.003 |
It can be seen from the table that if the theoretical minimum concentration is followed, most heavy metals can meet the 110-year discharge standard by relying only on the alkali precipitation method, and even reach the mainland's Table 3 standard for the metal surface treatment industry (ex: nickel <0.1ppm). However, it is actually impossible to achieve the theoretical concentration. The use of alkali precipitation method often faces the risk of excessive heavy metals for two reasons. The first is that actual wastewater treatment cannot reach the ideal state. No matter the pH adjustment, coagulation agent addition, solid-liquid separation and other procedures, it is impossible to optimize, and the theoretical concentration should not be achieved. Second, many additives are often used in each process. After the wastewater is mixed, the components in the water have become quite complex, and the various components are high and low and cannot be stable. For example, during the electroplating process, a wide variety of electroplating additives are dissolved into the wastewater. Under the interactive influence, heavy metal ions no longer simply exist in the water, but reduce the effectiveness of water treatment in various unknown forms. Therefore, when the wastewater composition is relatively complex, the alkali precipitation method is no longer a feasible and stable solution, and it is necessary to rely on other more effective treatment technologies, such as the recapture agent treatment method based on the principle of chemical precipitation.
Metal Sulfide Precipitation/ Heavy Metal Precipitation
This method belongs to the same chemical precipitation method as the alkali precipitation method, because there are many unknown interfering substances and chelating agents in the wastewater, which affect the treatment efficiency of the alkali precipitation method, and the recapture agent treatment method improves the problem of poor treatment efficiency . General heavy metal capture/capture agents are all sulfur-containing compounds. Quite a variety of heavy metal recapture agents can be seen on the market, each of which has a suitable heavy metal treatment category, and also exhibits final treatment effects under different pH conditions. The following five types of heavy metal collectors are compared,
| Type of HMP | Pro | Con |
|
Na2S based |
The most common recapture agent in the market is widely applicable, cheap, easy to obtain, and has certain effects on most metals. |
Hydrogen sulfide gas may be produced, which is detrimental to the on-site operating environment and is not conducive to employees and on-site operations. The effect of treating chemical nickel or chelated heavy metals is not good and cannot meet the discharge standards. |
|
SDTC/ Poly SDTC Blending |
It has excellent metal capture ability, and in the current use case, it can meet the most stringent Table III standard in the mainland (in which nickel must be less than 0.1ppm). | Still has a slight sulfide odor when operating at low pH. The treatment effect is poor for some metals. |
|
Poly Sulfide Based |
It has a wide operating pH range, low biological toxicity and light odor, and can be used as a substitute for sodium sulfide. |
The reaction time required is long and the effect on treating nickel and mercury in water is poor. |
|
TMT Based |
It can react with a variety of metals to form precipitation. It is fast and has high competitiveness. It has obvious effects on chelating agents such as EDTA or citric acid. | The treatment cost is relatively high, and the treatment effect on some metals is poor, such as cadmium, lead, zinc and mercury. |
|
Eco-Friendly Based |
It has a wide operating pH range and can replace the dimethyldithioamine-based type. It has no obvious odor during operation and reduces the public security risk of hydrogen sulfide. Excessive addition is less toxic to microorganisms in subsequent biological pools. |
It is not common on the market, is difficult to obtain, and the treatment cost is relatively higher than traditional recapture agents. |
In addition, for high-concentration waste liquid, the recapture agent can also meet the discharge water standard. However, it is generally recommended that for high-concentration nickel or copper waste liquids, you can first evaluate whether to use alkaline precipitation methods, oxidation methods, and pretreatment with recapture agents to reduce high-concentration heavy metal waste liquids to tens of milligrams per liter. Then enter the wastewater treatment system. This method can reduce the cost of processing high-concentration waste liquid, and can also avoid the situation where the wastewater treatment system is overloaded and the discharged water does not meet the standard.
Ion exchange resin (Ion-Exchange)
There are generally two concepts for using ion exchange resin. 1. It can be used for advanced treatment of heavy metal wastewater, especially when the metal concentration in the water is very different from the standard. The stable treatment capacity of ion exchange resin can be used as the final treatment unit. The second is used for high-concentration heavy metal waste liquid at the process end. Since it has not been mixed with other sources, relatively high-purity metals are concentrated into a concentrated liquid through ion exchange. The efficiency can reach about 70-80% recovery rate, and it is called The adsorbed metal is then desorbed by the recycling unit, and the unadsorbed resin is replaced to maintain system operation.
For advanced processing, it can target difficult-to-treat metal ions. Use ion exchange resin to exchange heavy metal ions in water to remove them. However, ion exchange resins are susceptible to poisoning. Taking the electroplating process as an example, after the electroplating tank, there are often multiple washes to clean the surface of the part. In order to maintain the quality standards of the workpieces, the washing water will be circulated and additionally replenished to achieve cleaning purposes, and the overflow water will be treated to remove contaminants. Metal salts used in metal processing are inorganic compounds that can be removed from water with ion exchange resins. During the ion exchange process, the resin exchanges hydrogen ions (H+) for positively charged ions (such as nickel) and hydroxyl ions (OH-) for negatively charged species (such as sulfate and chloride).
Electrolytic Process
This is one of the more mature technologies at present, and it is suitable for the method of recovery of heavy metal ions with high concentration. Precipitation of heavy metals reduced at the cathode by oxidation-reduction reactions is more suitable for high-purity and high-concentration metal waste liquids with simple components. However, this method is not suitable for the treatment of waste water at the end of the pipe, and the electrolysis efficiency can not reduce the concentration of heavy metals in the water to below the standard in addition to increasing energy consumption. Generally, the copper concentration of high-copper waste liquid from PCB printed circuit board factories is between 20,000-40,000ppm. If the recovery efficiency is calculated as 98%, the copper concentration of the treated waste liquid will still be 400-800ppm, and other back-end technologies must still be relied on. Only through treatment can the effluent standard of the law be met. Therefore, the electrolysis method is quite suitable for the pretreatment of high-concentration metal waste liquid at the front end. Through electrolysis, the benefits of copper plate recycling can be generated, and the initial and operating costs of the electrolysis equipment can be amortized.
.jpg)
▲The electrolytic recovery of high-concentration copper waste liquid into pieces of copper plates has become one of the commonly used solutions at present
Comprehensive comparison of various heavy metal treatment options
The following table organizes and compares the above four technologies in terms of processing limitations, operating costs and other characteristics.
| Alkali precipitation | Metal Sulfide Precipitation | Ion-Exchange | Electrolytic Process | |
| Raw wastewater metal level | 10-1000ppm | 0-100ppm | 0-10ppm | >1000ppm |
| System limit of metal level | <~5ppm | <~1ppm | <~0.1 ppm | <~100 ppm |
| Operation skill | Low | Medium | Medium | High |
| Initial Cost | Low | Low | Medium, Adsorption equipments were required | High, Need electrolysis equipment |
| Operation cost | Low | Medium | High | High |
| Sludge amount | High | Medium | Low | Low |
| Ingredients in water | Without Chelating Agents | Alloys, Chelating Agents | Alloys, Chelating Agents | Alloys, Chelating Agents |
| When to use | Suitable for wastewater with simple characteristics | Need to meet low emission standards | Requires fairly stable or extremely low emission standards | High concentration of metals with recycling value |
Each of these four technologies has its applicable scope and conditions. According to the characteristics and related parameters of each technology, the following selection process can be used as a basis for evaluation reference for you who are evaluating wastewater treatment solutions.
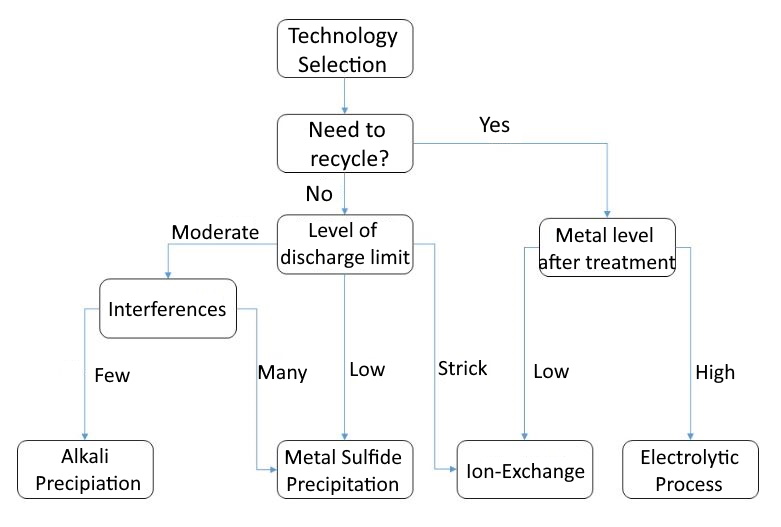
Here are two cases for reference.
Case A (recapture agent treatment + ion exchange)
Xiaoming is the person in charge of an electroplating factory, and there is a demand for heavy metal wastewater treatment, because the current treatment system is unstable, and the nickel cannot meet the standard stably. In response to the 110-year discharge standard, the nickel requirement is below 0.7ppm (the operating volume is greater than 150 tons/day) , wishing to evaluate other treatment options. According to the proposed process, first confirm whether there is a demand for nickel metal recycling. Since the concentration of nickel in the water is not high, there is no recycling benefit, so there is no need for recycling. Next, confirm that the metal nickel concentration in the discharge water is required to be below 0.7ppm. Compared with the required metal concentration, it is a low concentration, so the re-capture method is finally selected as the most appropriate treatment plan, and if it is necessary to achieve a lower concentration in the future You can consider adding another set of ion exchange equipment in the later stage to achieve stable standards.
Case B (alkali precipitation method + electrolysis method)
Lao Wang is the environmental safety supervisor of the PCB printed circuit board factory. He has two heavy metal wastewater treatment needs, namely high-concentration chemical copper wastewater and a large amount of medium-low concentration copper-containing wastewater at the process end. Because high-concentration chemical copper wastewater needs to be quantitatively diluted in the existing wastewater treatment system, the copper treatment effect is often mobilized and unstable. According to the scheme selection process, high-concentration chemical copper can be evaluated first because of its high concentration and high recovery efficiency. Therefore, recycling is considered. Electrolysis into copper plates has more potential for recovery, so electrolysis is selected for treatment. However, a large amount of low-to-medium copper-containing wastewater has no recycling benefits, and because chemical copper has not been injected into the wastewater treatment system, there are few interfering substances, and the copper concentration can be effectively treated below 3ppm by alkali precipitation.
Other information
Kelly chemical nickel removal and recapture agent operation test video:
CONTACT US
Kelly Chemical Corporation
Electronics
TEL:(02)2762-1985 ext 11200
Online Message
Leave your contact information,
and we will get in touch with you soon.
Email Consultation
After receiving your email,
we will process it as soon as possible.send Email